We keep an up-to-date list of all psychedelic clinical trials in our Trials Database.
Key Takeaways
- Ketamine is currently the only psychedelic in widespread clinical use. MDMA for the treatment of PTSD is currently in phase IIIb and is expected to be approved by 2023. Psilocybin for depression has just finished the data collection on a large phase IIb trial, of which a total 26 have been conducted or are currently ongoing.
- Most clinical trials are being conducted for depression (79) which affects between 2 to 6% of the world population (Our World in Data, 2017). The studies on suicidal ideation (associated with depression) are almost exclusively done with ketamine. There are 24 studies on PTSD with most of them being conducted with MDMA-assisted therapy.
- Studies in patient populations (phase II and later) are ramping up quickly with an average of 1,100 patients per year between 2016 and 2018 – more than doubling to 2,450 patients in clinical trials between 2019 and 2021.
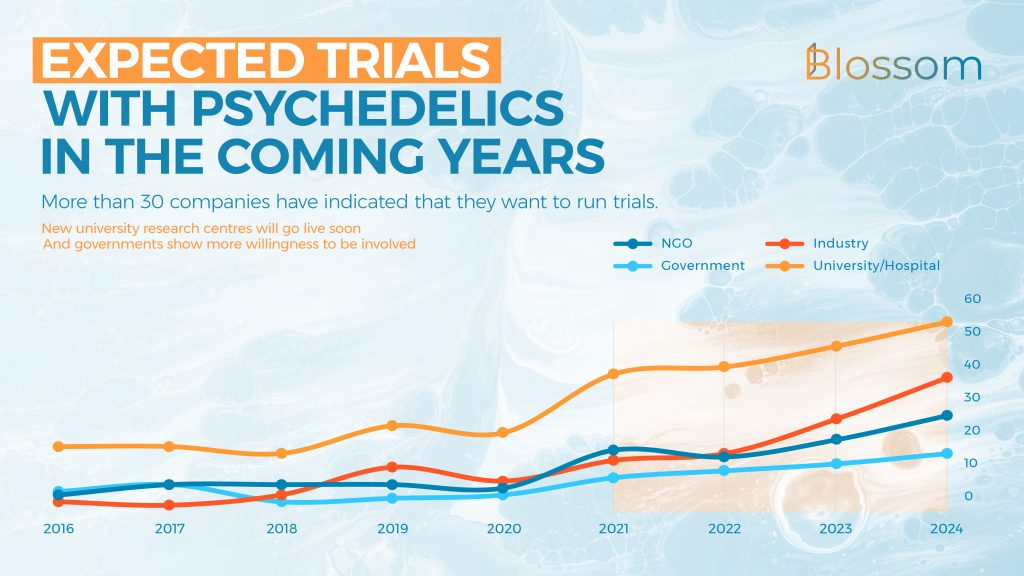
- Universities and hospitals commonly sponsor trials, and we predict that this will continue in the coming years. Companies have sponsored an average of 10 trials annually over the last three years, and we expect this to rise to 40 in 2024. More than 30 companies have indicated an intent to run clinical trials.
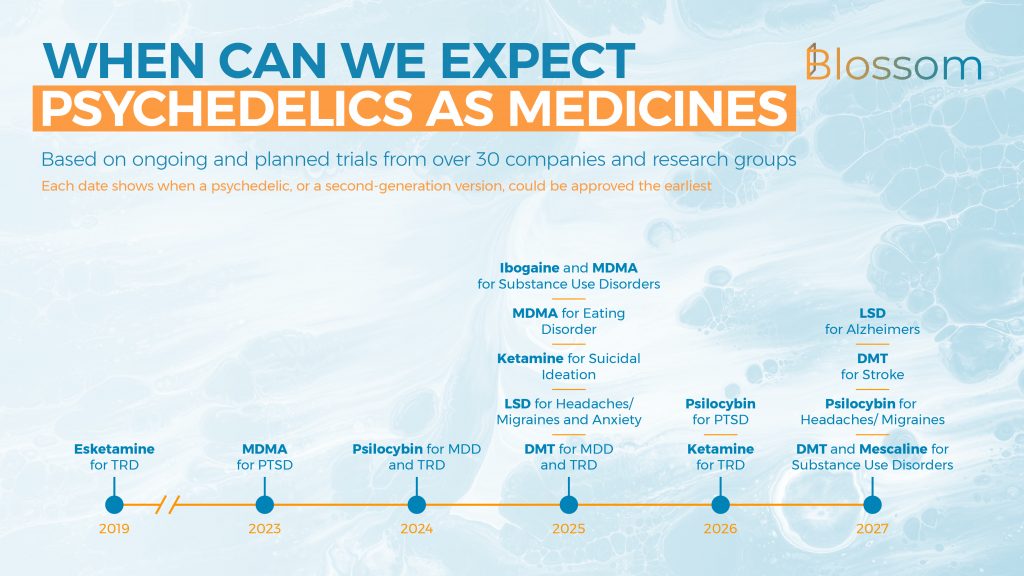
- We can expect MDMA-assisted psychotherapy for PTSD to be approved by the FDA in 2023. An optimistic timeline could see psilocybin for depression (MDD & TRD) approved the following year. Other approvals for ibogaine, LSD, DMT, and ketamine could follow in 2025.
Clinical Trials with Psychedelics
Studies with psychedelics have been ongoing since the 1950s and traditional use dates back several millennia. Millions of people around the world know how psychedelics can be a helpful tool in the betterment of mental health. From the Amazonian healing ceremonies to the Eleusinian Mysteries of Ancient Greece, people have sought to shift their perceptions of the world around them.
Building up on this knowledge, clinical trials are being used to understand – and demonstrate – the applications of psychedelics. This blog will take you on a tour through the clinical trials that are currently active or have been completed. These 305 studies*, to be exact, aim to generate data on dosage, safety, and efficacy.
If there are two take-home messages that I want to leave you with, it’s these:
- We are just at the beginning, there is still a lot of work to be done
- The work is accelerating, we are moving forward at an ever increasing rate
Index
1. A Primer on Clinical Trials
If you’re not too familiar or if you need a quick refresher, this section is for you. The goal of clinical trials is to generate data on dosage, safety, and efficacy.
Phase I trials are usually done with healthy participants to find the right or upper limit of dosage that is safe.
Phase II trials investigate how well the treatment works in a patient population, such as the Awakn MDMA for alcoholism trial. Phase III often compares the new treatment to the standard course of treatment, all while confirming effective dosing and effectiveness – such as MAPS’ MDMA-assisted therapy trial for PTSD. Often there are numerous studies contained within each phase.
Finally, phase IV trials monitor the longer-term response to the medication after launch. Nearly all of the studies of this type are currently being done with ketamine.
Of all studies listed, 124 are phase I trials, a third of the studies are phase II trials, and just under 10% are phase III trials.

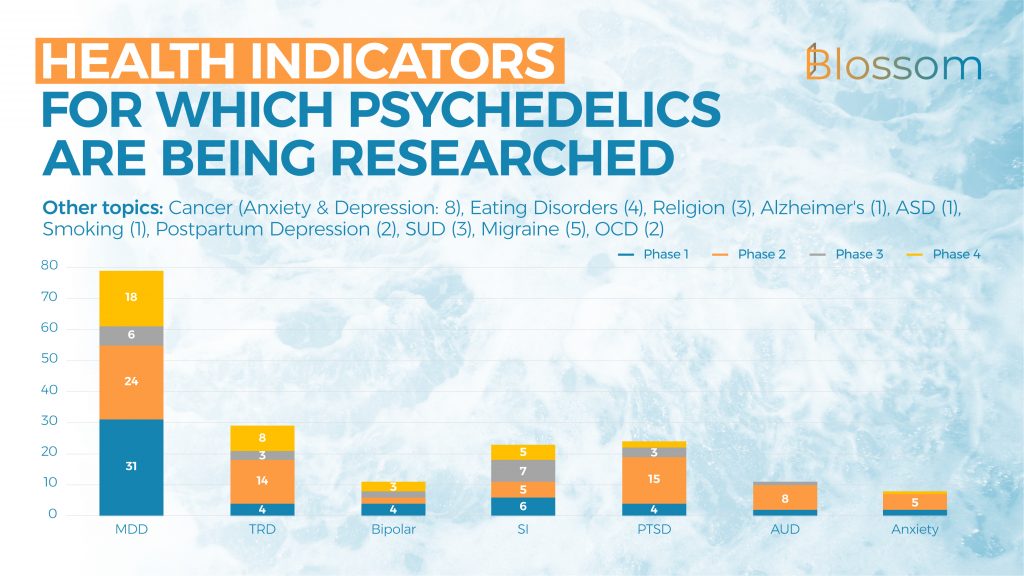
2. Health Indicators under Investigation
Clinical trials are being conducted for a wide variety of mental health indicators. Here the data, and thus we also, follow the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-V) which differentiates between a wide range of mental health indicators.
Although these are also called mental health disorders, it would be more correct to say that these indicators reflect abnormal behaviour without inferring judgment. Or put another way, there can be justified reasons for feeling depressed, for finding solace in drink, or dealing with trauma many years after the fact. Psychedelics offer the first substantive change in how we can help those suffering in decades.
Most studies focus on depression and the different ways that this is manifested. Most of the studies on those suffering from bipolar depression (both types) and suicidal ideation are currently being conducted with ketamine.
Next to the studies listed, these are the other topics that were the focus of the studies: Cancer (Anxiety & Depression: 8), Migraine/Cluster Headaches (5), Eating Disorders (4), Religion (3), Substance Use Disorder (3), OCD (2), Postpartum Depression (2), Alzheimer’s (1), Autism (ASD) (1) & Smoking (1)
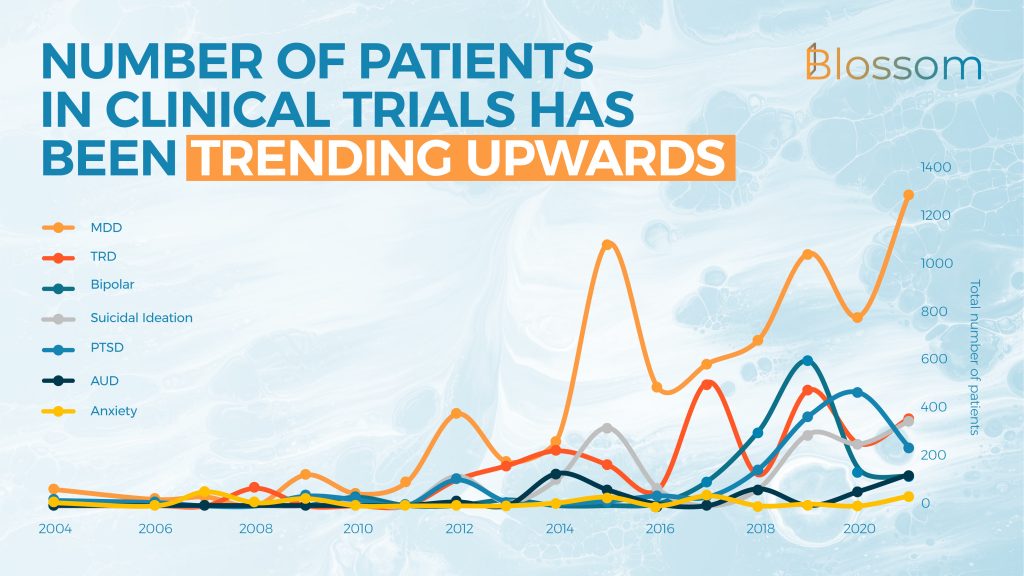
3. Timeline of Patient Population Studies
Over the years, we can clearly see a strong upwards trend in the number of patients being studied. Where an average of 1,100 patients per year were studied between 2016 and 2018, this more than doubled to 2,450 patients in clinical trials between 2019 and 2021.
The graph below has zoomed in on studies in patient populations and has excluded many of the phase I trials that are usually done with healthy participants. We have also excluded 3 large (500+) observational studies that are not representative of other clinical trials. A small number of patients are counted twice as they fit multiple categories (e.g. MDD and Anxiety).
The average number of participants in each trial is 52, which is higher than one might expect only knowing the most widely (pilot) studies from a few years ago. The numbers increase from 36 on average in phase I trials, up to 96 per phase III trial. Looking at the data without ketamine trials lowers the average in phase I trials to 30 patients per trial.
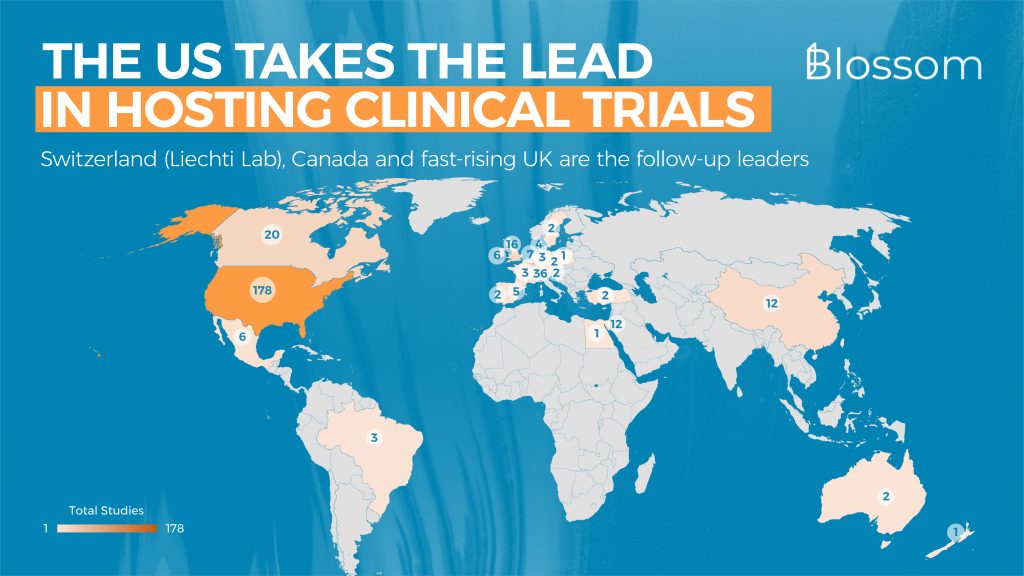
4. Map of Clinical Trials
Looking at geographic trial activity, America is the most active when it comes to running clinical trials, with Switzerland, mostly the Liechti Lab, coming in second place. The UK takes the fourth spot after Canada and has recently been ramping up the number of trials in the country.
Surprising is the number of trials in China, where ketamine has been researched for quite a while (since 2012). China has also conducted a study on psilocybin for the study of migraines.
Not only are most of the studies in Western Educated Industrialized Rich Democratic (also called WEIRD) countries, the patient populations are currently not that diverse. A review by Michaels and colleagues (2018) found that 82% of patients are white and only 2% are of Asian origin – a startling discovery, given this is where 60% of the world lives. Other ethnic groups are similarly underrepresented.
Studies on dosing, efficacy, genetics, and differing attitudes towards health services should be aimed at a more diverse and representative group for society, to better generalize the results of the current body of research.
The number of studies in the top 10 countries is as follows: United States (178), Switzerland (36), Canada (20), United Kingdom (16), China (12), Israel (12), Netherlands (7), Ireland (6), Mexico (6) & Spain (5).
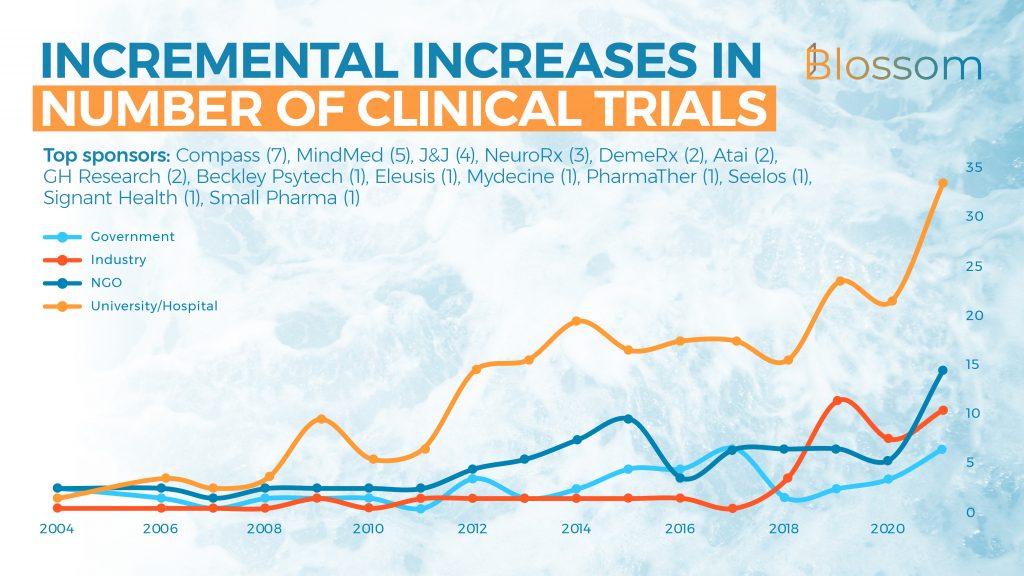
5. Timeline of Trial Sponsors
Press releases may have led you to believe that hundreds of trials are underway, it sure did so for me before our team dove into the data. The number of trials that have been sponsored has shot up in the last three years with an average of 10 trials being sponsored each year. Most of the current studies are being conducted by universities, who have recently also significantly increased their output.
The data for 2021 does include some trials that haven’t started yet, but we can expect some more to be registered before the year is over. At this time the biggest sponsors of research are Compass, MindMed, Johnson & Johnson, atai and its various subsidiaries.
Somewhat surprising is the number of trials already sponsored by governments. This is mostly done by the Veterans Affairs services in America. In the coming years, we expect this number to rise as psychedelic research becomes more mainstream, and more data is readily available. News from Germany and Australia show that more and more government funding (besides that already being received through universities) is on the way.
The current top industry sponsors of clinical trials are: Compass (7), MindMed (5), J&J (4), NeuroRx (3), DemeRx (2), Atai (2), GH Research (2), Beckley Psytech (1), Eleusis (1), Mydecine (1), PharmaTher (1), Seelos (1), Signant Health (1) & Small Pharma (1)
6. Timeline of Expected Trials
Projecting the current numbers into the future, we can expect the number of trials to continue to rise quite quickly. Not only should many companies actually execute on their plans to run trials, but we can also expect more studies to be sponsored by governments – such as the German government who is sponsoring the MIND Foundation’s psilocybin trial.
The commercial interest in research with psychedelics has only recently ignited. There are more than 30 companies that are either running or have run a trial (14), or have indicated that they plan to pursue a trial with a psychedelic. Although some of these press releases may not materialize in completed studies, many well-funded companies are on track to start clinical trials in the next few years.
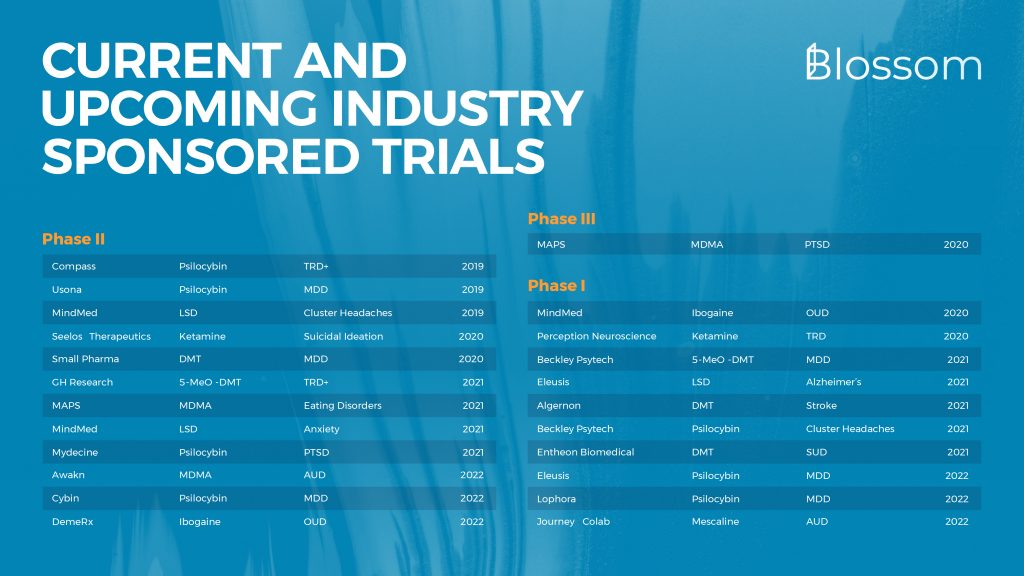
7. Industry Sponsored Trials
There are currently tens of companies pursuing trials of which the most certain are listed here. The duration to develop a psychedelic as medicine can vary widely. At this time we only have esketamine (Spravato, for TRD & SI) and MDMA for PTSD as examples.
With the very short development cycle and because it was a known chemical entity, the cost of development for Johnson & Johnson most probably is way below their average of $5.8 billion they’ve spent per newly developed drug. And presumably also below the average of $800 million that are the estimated costs to bring a single drug to market.
We do know that MAPS, although the longer timeline, is following a far leaner approach, with their total costs still under $100 million and with all the funds raised for FDA approval. Although the expected approval has crept up over the years, 2023 seems to be a very reasonable estimate.
The trials below are sorted by either their start date or probably start date if the trial isn’t ongoing right now. As previously mentioned, much research is picking up in the UK with Beckley, Awakn, and Small Pharma being three of the companies running trials.
Many more companies are running, or planning to run clinical trials. In alphabetical order: Algernon, Alvarius, Awakn, B.More, Beckley Psytech, BetterLife Pharma, Bexson, Brightminds, CaaMTech, Ceruvia, Compass, Cybin, Delix, DemeRx, Diamond Therapeutics, Eleusis, EmpathBio, EntheogeniX, Entheon Biomedical, Field Trip, Filament Health, GH Research, Gilgamesh, Journey Colab, Lobe Sciences, Lophora, MAPS, MindMed, Mindset, Mydecine, Neonmind, Perception Neuroscience, PharmaTher, Psilera, Psybio, Revixia, Sacred Medicines, Seelos Therapeutics, Small Pharma, Tactogen, Tryp Therapeutics, Usona, Viridia, & Wesana.
8. Psychedelics Approved as Medicines
“It’s tough to make predictions, especially about the future.” That is what famous baseball-playing philosopher Yogi Berra has said and with which I wholeheartedly agree.
The timeline here is our best, arguably optimistic estimate for when the different psychedelics or their second-generation equivalents could be approved for medical use.
This is based on the current drugs that are under development, such as MDMA for PTSD, and drugs that are just now going into clinical trials. For many compounds and health indicators there is good anecdotal evidence and we expect many to successfully pass through the trials.
Based on research from BIO, QLS Advisors & Informa UK from this year, the chances of developing a novel compound as a medicine are below 10%. Repurposing a drug for a new health indicator faces somewhat better odds and has about a 25% chance of getting approved.
Will this be different for psychedelics? Possibly. For many, we know the safety profile from earlier research and a lot of evidence is pointing towards effectiveness. But enthusiasm and personal transformation stories may not always translate to success in Phase III trials.
One final note that should be made here is the off-label use of psychedelics. It’s something that we see with the use of ketamine and that we can surely expect with other psychedelics. So even before some of these specific combinations will make it through the clinical trials, we can expect some to already be out there helping people.
9. Psychedelics as Medicines, Third Edition
As you have seen today, we’re still just at the beginning – the beginning of a huge change, and one that will continue to grow and blossom at an ever-increasing rate.
In the third edition of the ‘Psychedelics as Medicines Report,’ we will dive deeper into the data on clinical trials and many more aspects of psychedelics as medicines. Blossom is collaborating with PSYCH to release the third version of the report at the start of September this year.
The report will cover all aspects of this blossoming industry from clinical trials to patents, and discuss the potential of drugs, from LSD to salvia.
Leave your email below to be the first to receive it.

*Sources
Most of the data presented here today are courtesy of ClinicalTrails.Gov, an amazing resource that lets you search through all clinical trials registered with them. There may be some trials, and definitely some studies, that aren’t registered with them, and those aren’t included in the data presented.
If indicated, we have excluded some data such as large observational trials when looking at the data for the number of participants in the trials. We’ve also excluded studies that were withdrawn or terminated (almost always because of lack of funding). The date of each study is the date when the study has started or when they are planning to start. It’s usual for trials to take several years to complete, so keep in mind that data here is based on start dates.
You can find all our data and remix it yourself in this Airtable. Here you can also find some preliminary data on the (most certain to happen) trials that psychedelic companies have planned.
We are also grateful for the work done by Michael Haichin on Psilocybin Alpha that has tracked all the planned (commercial) studies.
Become a psychedelic insider
Get a Pro Membership to enjoy these benefits & support Blossom📈 full reports on Topics & Compounds
🧵 full summary reviews of research papers
🚀 full access to new articles
See Memberships

