To better understand the science of psychedelics, and how we can make the bridge to implementing them in practice, Blossom is conducting a series of interviews with psychedelic scientists. Our editor-at-large, George Fejer, speaks to Dr. Jay McLaughlin, Professor of Pharmacodynamics at the University of Florida, and one of the leading experts on kratom.
Key Insights
- Kratom and its active alkaloids are promising candidates to treat opioid addiction because they interact with different opioid receptor subtypes, which can counteract respiratory depression, opioid addiction, and its withdrawal symptoms.
- Salvinorin A is also an interesting candidate for treating addiction because it completely blocks out the rewarding effects of drugs, however, this is also accompanied by unpleasant subjective effects that could potentially cause drug relapse. Scientists are now developing multifunctional (partial) agonists, which initially block drug-related reward effects, followed by longer-term mitigation of withdrawal symptoms
- Mitragynine, and other alkaloids of kratom, seem to already have this type of multifunctionality. Not only do they activate opioid receptors, but they also work entirely different kind of receptor altogether called the α-adrenergic receptor, which is the target site of opioid withdrawal medication. This may explain why kratom alkaloids can substitute some of the effects of opioids and simultaneously treat withdrawal symptoms.

Author: George Fejer is a freelance scientific writer and a team coordinator of the ALIUS network for consciousness research. He studied Cognitive Neuropsychology (M.Sc.) and Bioscience (B.Sc.) and researched the effects of psilocybin microdosing on perception. His current work compares the phenomenology of serotonergic versus anticholinergic hallucinations using a mixed qualitative and quantitative methodology.

Expert: Jay McLaughlin (Ph.D.) is an associate professor of pharmacodynamics at the University of Florida. His research aims to identify the neurobiological systems underlying behaviour, characterizing them, and related psychological disorders to develop new therapeutic interventions utilizing molecular, pharmacological, anatomical, and behavioural methods. His projects encompass both basic science and drug discovery elements.
George Fejer
We were interested in your work because there is a lack of solid research on the effects of kratom (especially in Europe), but you and your colleagues at the University of Florida seem to be at the forefront of researching the active alkaloids of this plant-derived substance. Could you give us a brief introduction of your background and what led you to take an interest in investigating kratom and its active alkaloids?
Jay McLaughlin
I have a doctorate in neuroscience, and I am a behavioural scientist and pharmacologist. I’m interested in investigating both the basic mechanisms that mediate behaviour and treatments that try to help disorders of behaviour. I’ve done research on drug abuse for some time, and one of the questions that we’ve been interested in is trying to find better treatments for opioid dependence and opioid addiction.
As you may be well aware, there’s a significant amount of literature out there showing that kratom could be useful in treating addiction, or at least there are a number of people reporting that they use kratom to treat opioid physical dependence as well as to ease their cravings for opioid addiction. But there is a surprisingly small amount of data available, so I am trying to conduct more controlled studies to better understand the science behind kratom.
“Mitragynine, or other kratom alkaloids, may have that perfect combination of activity as a partial agonist of mu-opioid and α-adrenergic receptors.”
How kratom works
George Fejer
Interesting developments! So maybe we could drill in on some of the nuances of how kratom works. For example, kratom’s active component mitragynine also binds to the μ(mu) – opioid receptor just like morphine or heroin. However, mitragynine has far less addictive potential. Do we know what type of molecular properties are in play and what contributes to these differences in their addictive potential? How much do we know, and how much do we not know up to date?
Jay McLaughlin
We don’t know a lot and that is driving a lot of the further studies that are underway. What we do know is that kratom is a natural product, it is used as a tea, not unlike a mug of coffee in the morning. But in the case of kratom, it has somewhere in the neighbourhood of 54 alkaloids, specific small chemicals that are mixed in that natural product and that are expressed naturally in the plant.
We still don’t know a lot about each and every one of those alkaloids, so we continue our studies with Dr. McCurdy, Dr. Majumdar, and a number of other researchers. What I can tell you is that in the case of the primary alkaloid mitragynine is among one of the majority alkaloids in that natural mixture, and has become the subject of a relatively lot of studies.
“While kappa receptors produce good analgesia, they don’t cause respiratory depression, and they’re not addictive when they’re activated [ … ] so it’s possible that there’s a mixture of mu and kappa opioid activity in mitragynine that is counteracting some of the negative side effects and making these substances safer. “
As you pointed out, this is because it has similar mu-opioid agonist properties as morphine, but it may have some other unusual properties compared to that as well. Dr. Majumdar has demonstrated that it seems to have the ability to work as a partial agonist, or as a biased ligand. What that means is it may not engage in the same sort of signalling that morphine engages in when it interacts with the opioid receptor, with the consequence that it may be less addictive, and perhaps have fewer liabilities than what we see with morphine.
Secondly, there have been additional studies from a number of groups now that suggest that mitragynine, and maybe some of the other kratom alkaloids, may work at multiple opioid receptor sites, for instance at the Kappa opioid receptor. There are three kinds of opioid receptors generally accepted, the μ(mu)-opioid receptor, δ(delta)-opioid receptor, and the κ(kappa)-opioid receptor.
While kappa receptors produce good analgesia, they don’t cause respiratory depression, and they’re not addictive when they’re activated. They actually work against that, so it’s possible that there’s a mixture of mu and kappa opioid activity in mitragynine that is counteracting some of the negative side effects and making these substances safer.
And thirdly, there’s been some really cool recent evidence that suggests that mitragynine may also work at an entirely different kind of receptor altogether called the α-adrenergic receptor. And that’s interesting because both clonidine and lofexidine are treatments for opioid withdrawal that work on the same receptor site. They can ease a lot of the symptoms of opioid withdrawal, which are otherwise physiologically very severe.
What this may all add up to is that mitragynine, or other kratom alkaloids, may have that perfect combination of activity as a partial agonist of mu-opioid and α-adrenergic receptors. At least, that is a working theory that we are currently investigating.
Strange opioid receptor interactions
George Fejer
It is indeed very interesting to note that there are three different opioid systems in place, and this may lead to problems with public perception of substances that act differently on the opioid system with its connotation derived from the addictive substance opium. But as you mentioned in your previous answer, there is a lot of complexity when it comes to figuring out what is going on under the hood.
For instance, Valentino and Volkow (2018) have used the infographic (adapted below) to illustrate that although all three receptors have analgesic properties, they seem to be positioned on diametrically opposed ends of mood and hedonic continuums. Kappa-opioid agonists, as opposed to mu-opioid agonists, produce dysphoria, stress, and negative affect, and delta-opioid agonists have anxiolytic and antidepressant activity.
Isn’t it strange that kappa-opioid agonists produce negative affect, stress, and dysphoria but seem to have an anti-addictive potential? What can you say about the relationship between these three opioid receptors and how do you determine which of them to target for the treatment of mood disorders?
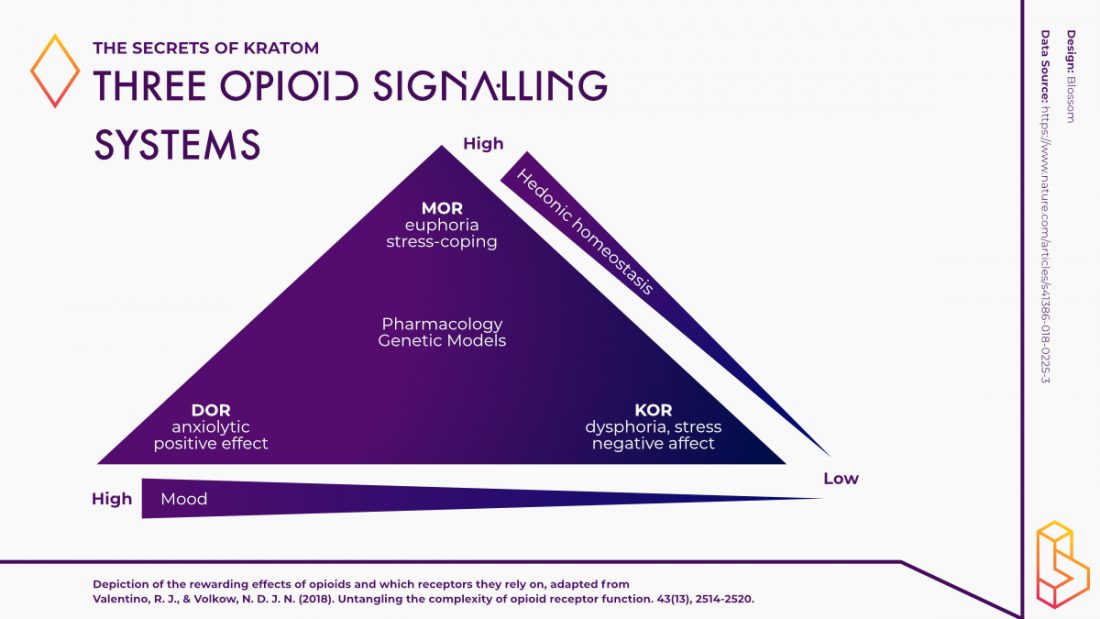
Jay McLaughlin
I can make this even more complicated for you, because actually all three of these receptors, mu, kappa, and delta, are genetically, structurally, and functionally very similar to one another. What we know from Lutz and Kieffer (2013) is that all three of these opioid receptors are connected to inhibitory signalling processes, they basically serve to make neurons less excitable, i.e. less likely to produce action potentials.
So, you might as well ask, how is it something that that shares such a similar system can have such different behavioural effects? And the answer to your question is simply is location, location, location, just like real estate. A number of these opioid receptors are located throughout the body and that accounts for a lot of the differences in their actions.
Mu opioid receptors are located on a system that regulates the dopamine reward pathway and basically increases its signalling whenever something positive happens. That dopamine reward pathway serves as a coincidence detector, telling your brain whenever something good happens, and to want more of it.
Essentially most drugs of abuse hijack that pathway to increase dopamine unnaturally to very high levels, and that is thought to underlie a lot of what makes substances reinforcing. This is not the full answer, but it’s straightforward enough for our purposes today.
Mu opioid receptors are found on, among other things, the inhibitory GABA interneurons that shut down that dopamine reward pathway. So if you shut down those inhibitory GABA interneurons, the dopamine system is going to increase its firing rate, and it’s going to be reinforcing drug-seeking behaviour.
A commonly used analogy to explain this is: when you are driving a car if you take your foot off the brakes you will go faster as your car drifts forward. And we think that is how a lot of natural rewards work since beta-endorphins are released in response to natural reward work through mu-receptors to naturally signal that effect. But the signal only lasts a few minutes because beta-endorphin is very quickly metabolized, whereas morphine is metabolized relatively very slowly so its effects can last quite a while. Substances such as heroin can also have this long-term reinforcing effect.
Now, kappa receptors are actually located on those dopamine neurons themselves, in areas that basically allow you to shut off the dopamine neurons. So they are directly inhibiting the reward pathways. We think that’s what’s underlying a lot of those negative side effects or kappa agonists, such as dysphoria, stress, and negative affect.
Dynorphin peptides naturally activate the kappa receptor system to serve as a check and balance against this reward system. But if you put in a natural kappa-opioid agonist like Salvinorin A (or a synthetic one like U50,488) it will actually suppress that dopamine pathway in ways that can be directly measured through various different biochemical assays. And behaviorally those animals actually show dysphoria, they show conditioned place aversion, they show the opposite of reward behaviour.
We know that stress works through this, because stress causes the release of dynorphin (among other things), and we know that long-term stress seems to have an impact through the kappa systems to do much the same thing. Negative affect has also been a hot topic as a number of researchers have suggested that mood disorders, such as depression and anxiety, are also mediated by activity through that same kappa receptor.
Hopefully, that casts a little light as to what’s going on there!
Helping those with addictions
“Over the last 10 years, there has been a realization that maybe we can benefit from drugs that work at multiple sites, or maybe have less than full efficacy at one receptor site so that we can avoid some of the side effects that come with continued activation.”
George Fejer
Do you think that these negative affect states are conducive to helping people with addiction?
Jay McLaughlin
That’s actually a really good question and there is a lot of debate about that. The early work of great scientists such as Toni Shippenberg and others demonstrated that we can give a kappa agonist to an animal, and completely block the rewarding effects of almost anything (Shippenberg, Herz, Spanagel, Bals‐Kubik, & Stein, 1992; Spanagel, Herz, & Shippenberg, 1992).
Cocaine, heroin, you name it. We can stop it with the Kappa agonism. The problem is a when you take that to the clinic, people will not take them because they find those drugs too dysphoric. At least the normal Kappa agonists. And there’s a lot of research to try to figure out better ways to get around that problem via other analogues.
Secondly, it’s been found that the dysphoric effects of the Kappa agonist can drive the patients back to seeking drugs that counteract these effects. For instance, if a patient is already maybe abstinent from opioid use, you might want to avoid inducing negative states that might trigger a relapse. So that is another limitation to consider, but people are working on a solution through compounds that have both agonist and antagonist properties.
For instance, a partial agonist may initially suppress the rewarding effects of drug abuse, but can then act as an antagonist to protect against the stress effects that might promote abstinence over time. George Koob and others including myself have been trying to look for that right profile of activity that will be the most well-tolerated during treatment.
George Fejer
In terms of therapeutic paradigms, much of the older treatments for addiction were aimed at substitution. That is to say that the addicted patient would try to taper off the substance of abuse by replacing it with another one that mitigates the withdrawal symptoms.
But now there is a lot of discussion around the concept of disruptive therapeutics, whereby patients receive a substance such as psychedelic tryptamines, ibogaine, or ketamine, with the aim of restructuring addictive patterns of behaviour. Recently, a study by Doss et al. (2020) conducted at John Hopkins University has shown that Salvinorin A enacts a restructuring of resting brain activity that is many ways similar to that of psychedelics.
However, many researchers are opposed to integrating Salvinorin A because of its hallucinatory effects that are considered by many people as unpleasant. But on the other hand, for many patients, the subjective effects of intravenous ketamine treatment are also not necessarily pleasant. What do you think about the prospects of integrating Salvinorin A as a disruptive intervention for the treatment of addiction?
Jay McLaughlin
The Kappa agonists possess problems, but the Kappa partial agonists have some promise, and this gets us back to the idea that drugs work at multiple sites. Over the last 50 years, there has been an evolution in scientific research and drug discovery, where we’ve been trying to find drugs that were increasing selective with high efficacy at one specific site.
But over the last 10 years, there has been a realization that maybe we can benefit from drugs that work at multiple sites, or maybe have less than full efficacy at one receptor site so that we can avoid some of the side effects that come with continued activation.
With kappa agonism that is particularly relevant. Kappa partial agonists may give you more benefit if you have a mixture of activities where you are initially suppressing dopamine, but then antagonizing that site over time to protect against stress and other negative side effects of long-term Kappa-opioid agonism.
The idea here is to block the initial cravings and reinforcement of drug-seeking behaviour through the suppressive effects of kappa agonism on the dopamine reward pathway long enough to get over the critical moment of wanting to use it, and then have a longer-term abstinence-promoting antagonism kick in. George Koob has done some terrific work on that.
Salvinorin A is a full Kappa agonist — in fact, it’s probably one of the most potent agonists we know; Brian Roth has demonstrated this some years ago. Salvinorin A clearly suppresses the rewarding effects of drugs abuse, but animals find it incredibly aversive. We typically measure aversive behaviour by testing with the Conditioned Place Preference assay, where mice are put into a cage with two compartments to observe how much time they spend in an environment that they associate with positive or negative feelings when experiencing the subjective effects of the drugs in specific compartments.
When you are conditioning animals with Salvinorin A, you actually have to close the tops of those boxes because the mice immediately want to jump out the top. To give you a sense of how much they want to avoid this drug, in our apparatus, it would be the equivalent of you jumping over a four-story building to escape the negative sensations.
And although many people use this drug recreationally to experience its psychedelic effects, the negative components of its effects suggest that it may be more difficult to work with for the treatment of drug abuse.
Potential new analogues
George Fejer
Are there any new analogues that hold promise to be better for treatment than Salvinorin?
Jay McLaughlin
Jane Aldrich at the University of Florida is currently working on peptide-based cyclic tetrapeptides that possess this multi-functionalism, working as Kappa agonist or partial agonist and antagonist. She is using the structure-activity relationships learned from her library to tailor the construction of those compounds to optimize their therapeutic effects.
Susruta Majumdar has also been using molecular modelling to design structures that act as a low efficacy opioid compound and a partial agonist to optimize its analgesic benefits while maximizing its ability to block addiction without liabilities. We have already tested some of those compounds, and the animals don’t show respiratory depression or conditioned place preference. Those compounds also effectively wean subjects physically dependent on morphine off the opioid without withdrawal
George Fejer
Those sound like promising developments! But in terms of bringing these substances into clinical trials and making them more accessible, do you think we should be focused on researching pure alkaloids, optimizing analogues, or testing whether plant-based preparations, such as kratom or Salvia Divinorum, are also viable treatment candidates?
Jay McLaughlin
All of the above! It’s a good question, with multiple parts to the answer.
There are a lot of kratom users with estimates of as many as 50,000 or 60,000 people who are using kratom daily to ease their cravings and avoid withdrawal. You can’t argue with results, so if it’s actually helping people to stay off of illicit opioids, that’s a good thing. But we don’t know which of those 54 alkaloids in that kratom mix is actually causing that effect.
Research often starts with the natural products (or here, the alkaloids inside kratom) and then identifying things that they can improve on to give it better pharmacokinetics. For instance, better druggable properties that make it orally active. Work continues and that’s where it starts to move into the synthetics and testing new drug candidates.
My colleagues Chris McCurdy and Jane Aldrich are doing just that. Some of this is starting to move into advanced Pre-Investigational New Drug (Pre-IND) work, which entails trials with monkeys and things like that. So we are getting very close to being able to put forward clinical trials.
Jane Aldrich has already submitted a proposal to the federal government to commence Phase I clinical trials with her cyclic tetrapeptides. That work is underway, and I think there are a lot of promising leads in there. With hope, we will find some better treatments that can help people out.
But all of that costs money, so it also depends on how we can balance funding priorities and research needs versus the budget. I’m lucky enough to work with, now and having trained under, a lot of talented people working on this. But the need to find effective treatments for the opioid epidemic is becoming increasingly dire.
We’ve had a record number of opioid-related overdose deaths in the last year alone, with reports here in the US on National Public Radio (NPR) this morning that it has gotten worse since the numbers reported back in July. We don’t see an end in sight right now, so new treatments and more importantly, telling people what that entails, is going to be really important for getting them some help. So thanks for taking the time to do this interview. It means a lot!
References
Doss, M. K., May, D. G., Johnson, M. W., Clifton, J. M., Hedrick, S. L., Prisinzano, T. E., . . . Barrett, F. S. J. S. r. (2020). The acute effects of the atypical dissociative hallucinogen salvinorin A on Functional connectivity in the human brain. 10(1), 1-12.
Lutz, P.-E., & Kieffer, B. L. J. T. i. n. (2013). Opioid receptors: distinct roles in mood disorders. 36(3), 195-206.
Shippenberg, T. S., Herz, A., Spanagel, R., Bals‐Kubik, R., & Stein, C. J. A. o. t. N. Y. A. o. S. (1992). Conditioning of opioid reinforcement: neuroanatomical and neurochemical substrates. 654(1), 347-356.
Spanagel, R., Herz, A., & Shippenberg, T. S. J. P. o. t. N. A. o. S. (1992). Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. 89(6), 2046-2050.
Valentino, R. J., & Volkow, N. D. J. N. (2018). Untangling the complexity of opioid receptor function. 43(13), 2514-2520.
Become a psychedelic insider
Get a Pro Membership to enjoy these benefits & support Blossom📈 full reports on Topics & Compounds
🧵 full summary reviews of research papers
🚀 full access to new articles
See Memberships

