KEY TAKEAWAYS
Medical psychedelics companies are valued at over US$10 billion.
The market value of medical psychedelics is valued at US$190 million and expected to exceed US$2.4 billion by 2026.
The pandemic has great potential to rattle the mental health landscape, and psychedelic therapy offers another tool to support the alleviation of a growing global epidemic.
Blossom & PSYCH
In The Psychedelics as Medicine Report, we reported on the size of the psychedelic market. The market is growing at an exponential rate and has seen much activity on both sides of the pond. Although the current revenue (mostly from ketamine-assisted therapy) is relatively small, there are high expectations and associated valuations for psychedelic companies.
A Growing Market
The market opportunity in the nascent and growing psychedelics’ space is being harnessed by a growing base of institutional investors seeking to establish and scale businesses in a landscape showing scientific and commercial promise.
While capital invested in psychedelic companies is over US$2 billion, the current value of medical businesses surpasses US$10 billion, with atai Life Sciences’ initial public offering (IPO) earlier this year making it the most valuable publicly traded business among the 47 publicly traded businesses in the sector, currently valued at US$2.4 billion (as of September 2021).
Biotech and pharmaceutical companies continue to dominate businesses within the sector, though there is a growing base – and appetite for – research clinics, therapy providers and software infrastructure. Canada’s capital markets remain a popular choice for psychedelic companies.
There are over 100 ketamine clinics in operation in the USA with many more in the pipeline. Despite gaining regulatory approval in the UK, Johnson & Johnson’s Spravato (esketamine) did not receive approval by NICE (National Institute for Health and Care Excellence, which approves drugs for use by the National Health Service) twice, citing the cost-effectiveness estimates for esketamine to be much higher than the cost-effective use of National Health Service (NHS) resources, and would like to better understand the long-term use of esketamine in patients, and whether the improvement in symptoms and quality of life can be sustained following treatment. Spravato costs about £10,000 (US$14,000) per course of therapy. In the USA, it was suggested that the price of Spravato needs to be reduced by 40% in order to be cost-effective for the management of treatment-resistant depression (TRD).
Approval Landscape
While ketamine has been available as an off-label drug for depression for many years, it wasn’t until 2019 when Spravato (esketamine, a ketamine derivative) got FDA approval, and the following year approval by the European Medicines Agency (EMA), for TRD, or for use in psychiatric emergencies. Currently, MAPS‘ MDMA-assisted therapy for PTSD is the only psychedelic therapy undergoing phase III trials with commercialisation goals; globally, there are 82 active phase II trials with psychedelics, of which 45% are completed. Among the phase II trials with commercialisation goals, Usona and COMPASS Pathways are investigating psilocybin therapy for the treatment of depression. The research path for many aspirational researchers will take years to go through the various stages of clinical trials, analysis and reviews, and as such, we have focused primarily on ketamine, psilocybin and MDMA, as these compounds are either being used for treatment already or are close to being reviewed for widespread therapeutic purposes.
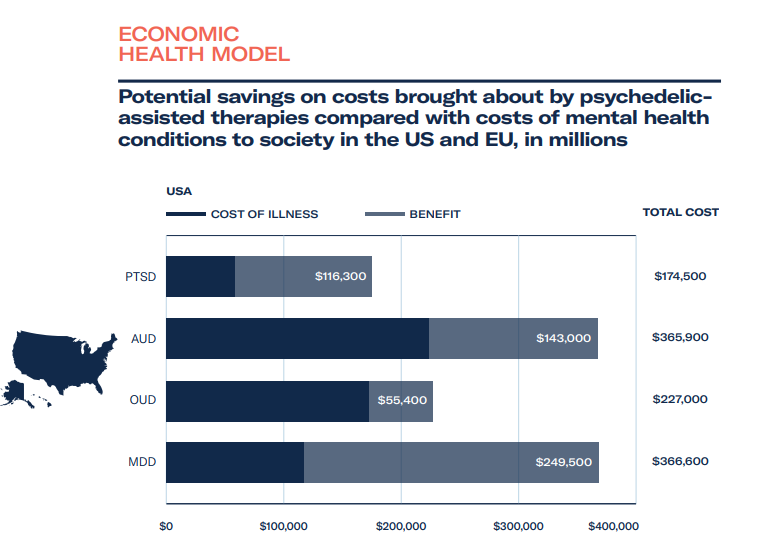
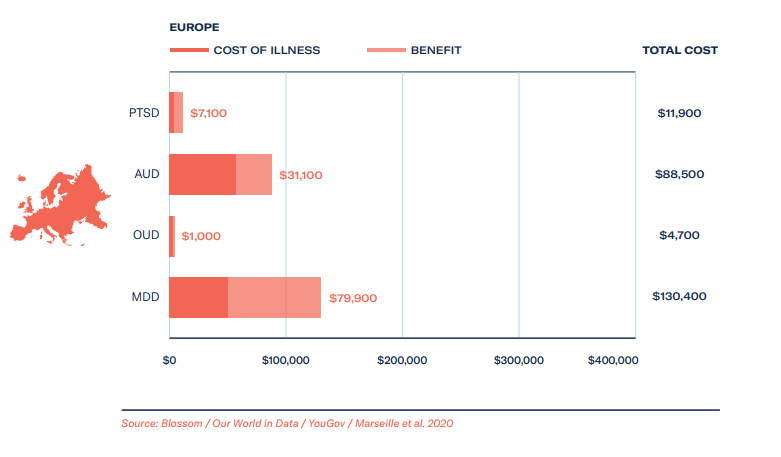
Based on estimates around MAPS’ therapy goals during their data exclusivity period from 2023-2029, we forecast total healthcare savings to be rather substantial for patients – at least US$18 billion, and revenue gains from providing therapy and training to be in the region of $7 billion. Assuming a linear growth rate for ketamine clinics, at US$600 per course of treatment, the revenue of ketamine clinics could be upwards of US$3 billion over the same period, with healthcare cost savings on a par with revenue gains.
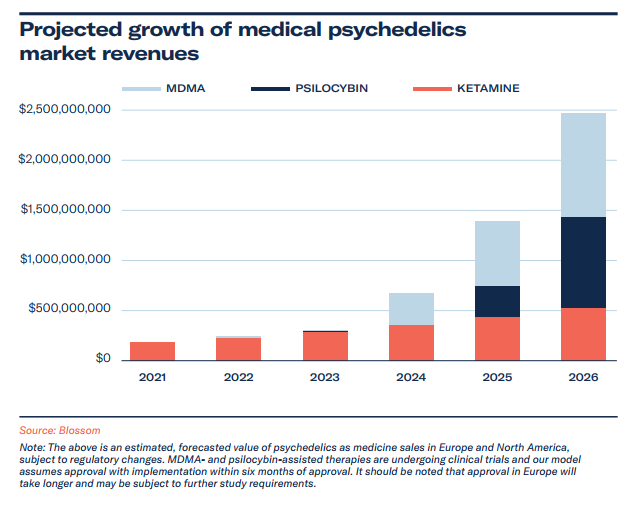
Projected Growth of Medical Psychedelics Market
In modelling the medical psychedelics market, we focused on therapy training and services, to encapsulate the current market condition. In 2021, with ketamine the only compound used off-label widely for the treatment of mental health conditions, and esketamine for TRD, ketamine-assisted therapy dominates the market. We expect compassionate use to increasingly include psilocybin and MDMA, with policy and health advocates being strong proponents of such therapies. MDMA is currently available for compassionate use in Israel, Switzerland and Canada. Compassionate use exception is expected in 2022 for psilocybin-assisted therapy in Europe, with Canada’s section 56 exceptions being another route to early access before Health Canada’s consideration.
Our model is dependent on the exact dates of approval by the FDA or equivalents in other countries. Nonetheless, the model incorporates data on therapists’ availability (both who is trained and how many patients they can serve), consumer willingness, and adjacent revenues such as therapist training and other complementary therapy services (e.g. Wavepaths’ music service). Underground and non-medicinal revenues are not modelled.
Psilocybin therapy (and specific training protocols) will grow in sales through clinical access being granted to practitioners (beginning in early 2023) – and we expect this to be a key driver in societal education and contributor to wider reforms across America. We predict MDMA-assisted therapy to take a strong market share from 2024, owing in large part to the later trial stage, plans to create therapy opportunities, and MAPS’ headstart to therapist training.
There are many companies researching best practices for the training of psychedelic therapists, who have distilled these into training programmes, such as the MIND Foundation and COMPASS Pathway. Diversity in the offerings provides aspiring therapists with options from which they could choose training most aligned to their goals, though it will be encouraging to see more government subsidies and universities embed psychedelic therapy training, to increase access – both in terms of knowledge and resources – and widen therapist potential for the growing demand, with
more PATs expected to be approved in the near future.
The Psychedelics as Medicines Report continues the reporting with insights on:
- The cost of illness, how much are we currently spending and can we save if psychedelic-assisted therapy is approved
- Mental health during Covid-19 and how this has influenced our cost-model
- Information on legislation and all other topics that cover psychedelics as medicine
Become a psychedelic insider
Get a Pro Membership to enjoy these benefits & support Blossom📈 full reports on Topics & Compounds
🧵 full summary reviews of research papers
🚀 full access to new articles
See Memberships

